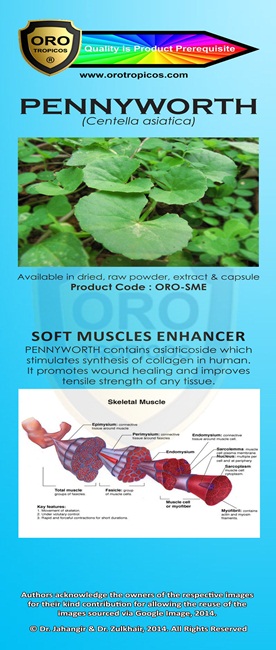
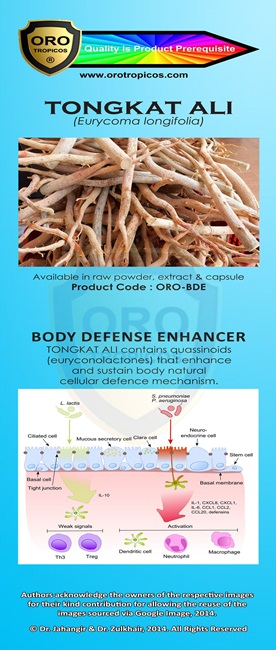
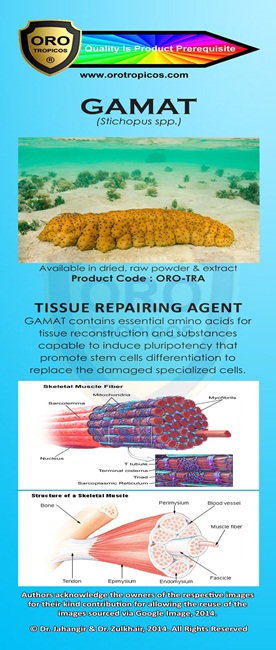
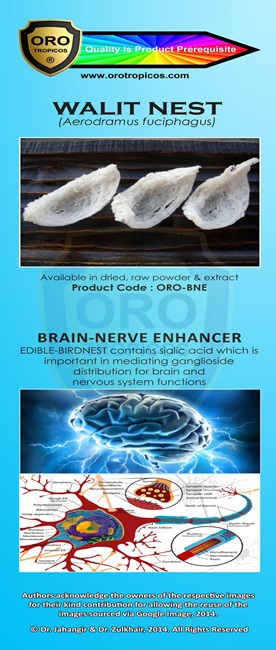
Kami adalah ONE STOP CENTRE untuk PENYELESAIAN PERNIAGAAN anda.

Anda mungkin pernah mendengar bahawa beberapa syarikat Malaysia mengalami kerugian besar kerana produk herba mereka diarahkan oleh pihak berkuasa untuk dikeluarkan dari penjualan di Amerika Syarikat, Turki dan Malaysia kerana didapati mengandungi bahan kimia terlarang dan berbahaya.![]()

Kami menawarkan minyak pati dari ramuan berharga dengan aroma yang menyenangkan bagi menenangkan fikiran dan keadaan seharian anda. ![]()

Kami menyediakan produk siap / ready stock OROTROPICOS yang terbaik dan anda boleh membuat pembelian secara di atas talian dengan hanya di hujung jari anda.![]()

Pelabelan produk anda bermaksud produk anda selamat atau tidak beracun dengan menyatakan kepada pengguna bahawa produk anda telah diuji bagi membuktikan tuntutan tersebut.![]()
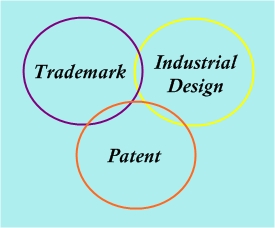
Memang benar bahawa bilangan syarikat PKS baru terus bertambah tetapi malangnya, jumlah penduduk pekerja Malaysia TIDAK meningkat pada kadar yang sama. Oleh itu, akan ada persaingan yang ketat dengan pelbagai taktik pemasaran untuk mengekalkan penjualan. Sebilangan perniagaan secara terbuka akan menyalin atau meniru nama, logo, label, pembungkusan syarikat anda dan bahkan mencuri formula atau kaedah anda.![]()
 English
English Bahasa Malaysia
Bahasa Malaysia